|
|
Free Silicone Injections
General Considerations
- Commonly used for breast augmentation in the ‘50s and ‘60s
- It was stopped because of safety concerns and it was ineffective
- Eventually, banned by the U.S. Food and Drug Administration in 1992
- However, it may still be found in older patients and immigrants, especially from South America and Asia
- Complications are common and include inflammation with
- Formation of silicone granulomas
- Fibrosis
- Lymphadenopathy
Clinical Findings
- Silicone granulomas are clinically palpable
- Diffuse nodularity and hard lumps, making manual breast examination difficult
Imaging Findings
- On mammography, free silicone demonstrates multiple, very dense and lobulated masses throughout the breast with or without peripheral calcifications
- The masses can cause distortion of the breast parenchyma and obscure visualization of a small breast cancer
- Extremely dense lymph nodes may also be present
- Sonographic and MRI findings are similar to those of extracapsular implant ruptures
- But, findings are scattered throughout the breasts without the presence of an envelope or fibrous capsule
- MRI, especially with fat and water suppression technique, will afford optimal visualization of free silicone and should permit differentiation of free silicone from a breast neoplasm
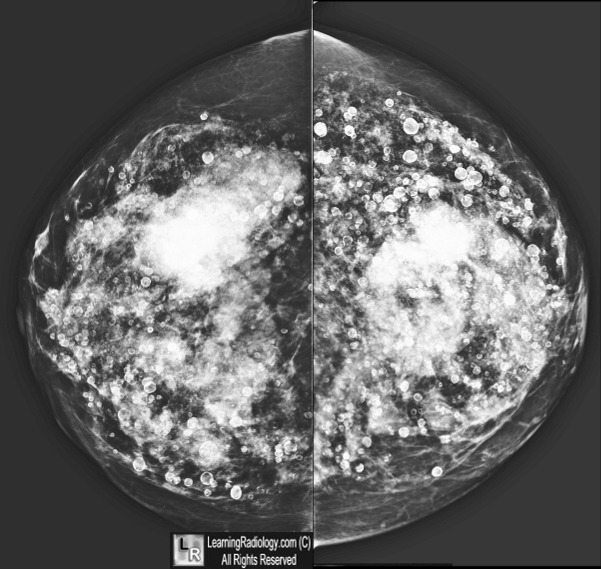
Free Silicone Injections, Breast. Craniocaudad views of both breasts show innumerable very dense and lobulated masses throughout both breasts
Challenges in Mammography: Part 2, Multimodality Review of Breast Augmentation—Imaging Findings and Complications. S Venkataraman, N Hines, and P Slanetz. AJR. December 2011, Volume 197, Number 6
|
|
|